Ethylene Measurement Applications in Agricultural and Horticultural Production and Research
29th Jul 2021
Ethylene is a phytohormone that is naturally produced by agricultural and horticultural plants at nearly all stages of their lifecycle. Managing plant response to ethylene has grown into a crucial commercial industry, as the hormone can have both negative and positive impacts on the production of agricultural and horticultural crops. Not surprisingly, there are precision tools that have been developed to detect even trace amounts of this gaseous hormone.
Ethylene in Plants
The gaseous nature of ethylene (C2H4), which is odorless and colorless, allows it to travel freely from its source by diffusion within a plant and also into the surrounding environment. Since it is volatile, ethylene is not stored or broken down by the plant. The hormone is necessary for the development of plants, but it can also spoil quality, depending on which stage it is produced in the supply chain; see Figure 1.
The agricultural and horticultural importance of ethylene production by plants is due to its role in the following:
● Promotion of flowering in fruits and vegetables
● Ripening in climacteric fresh produce
● Germination of seeds, for example in groundnuts
● Sprouting of tubers in potatoes
● Development of the root system, including adventitious roots
● Elongation of the stem through the growth of internodes and petioles, which is manipulated in rice seedlings
● Senescence and abscission of leaves, flowers, and fruits
● Plant response to stress as a signaling molecule
Ethylene is biologically active, and concentrations of just 0.01 ppm are enough to trigger physiological responses in plants. Climacteric fruits both need and produce ethylene amounts far exceeding this level during ripening. Besides plants, ethylene is produced as a result of combustion in machines and vehicles. Concentrations of 0.7 ppm ethylene produced by combustion engines in vehicles in busy motorways have damaged nearby vegetation. It has been shown to be harmful to humans at concentrations above 1000 ppm.
Ethylene measurements are necessary at preharvest and post-harvest stages, depending on which ethylene response stakeholders want to control. These are briefly discussed below. The most popular application is during the artificial ripening of fresh produce.
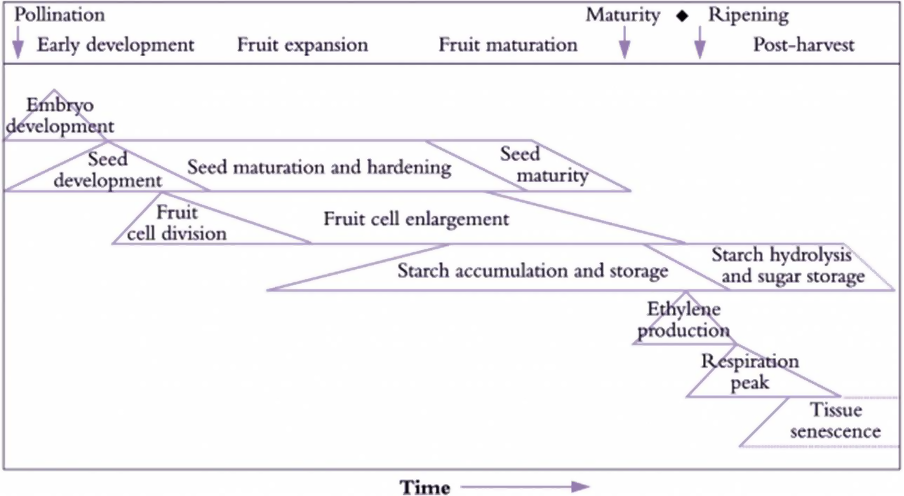 Figure 1: Ethylene production during climacteric fresh produce development and ripening. (Original diagram courtesy of I. B. Ferguson). (Image credits:
http://www.asps.org.au/wp-content/uploads/Chapter...
Figure 1: Ethylene production during climacteric fresh produce development and ripening. (Original diagram courtesy of I. B. Ferguson). (Image credits:
http://www.asps.org.au/wp-content/uploads/Chapter...
Indoor Farming
Cultivating vegetables and flowers indoors is becoming increasingly popular in an effort to meet year-round demand for several horticultural commodities. This can happen in greenhouses, vertical farming installations, hydroponics, and aeroponics. The internal environment of indoor farms has to be monitored and controlled, as the closed spaces build up gases such as ethylene or deplete gases like carbon dioxide and oxygen. These are problems that do not exist in open fields.
Ethylene can be produced by living plants or decaying waste. Many of the machines and equipment used in indoor farms could also produce ethylene, especially if they burn fossil fuels. Cigarette smoke can also be a problem, as minute concentrations of 0.05 ppm of ethylene can cause senescence and abscission of leaves and flowers, affecting the final yield. Many people use indicator plants to detect low amounts of ethylene, but fresh plants have to be used for each measurement when monitoring is carried out over longer periods and is not quantifiable.
It is easier to use sensitive devices that can take frequent and precise measurements of ethylene. Not surprisingly, the technology utilized in indoor farms was worth US$14.5 billion in 2020, and it is expected to increase by 9.4% to reach $24.8 billion by 2026. Post-Harvest Monitoring of Quality There are many uses and applications of ethylene measurement other than monitoring ripening, given that this phytohormone is involved in many crucial physiological processes in a plant. It is also used in quality control and disease detection. Ripen Fresh Produce Climacteric fruits, such as apples, mangoes, and bananas, are harvested when they are physiologically mature, but unripe. They can ripen postharvest with the help of artificial ethylene so that the carbohydrates stored as starch are converted to sugars during ripening.
Suppliers use artificially produced ethylene, in combination with specified temperature, oxygen, and carbon dioxide levels, to provide ideal conditions for ripening climacteric fruits. The amount of ethylene introduced has to be precisely monitored during the entire process to maintain levels of around 100 ppm, requiring sophisticated devices.
Whether the ripening is carried out in simple storage or sophisticated ripening rooms, the monitoring of ethylene remains a vital aspect of the process.
Control Quality to Increase Shelf-Life
Non-climacteric fruits, on the other hand, do not use ethylene for ripening, and carbohydrates are accumulated not as starch, but as sugars. Hence, they are harvested when mature and ripe, as ripening postharvest is not possible, as is the case with strawberries, peas, eggplants, etc.
However, the non-climacteric fruits are still sensitive to the ethylene effects of senescence and rotting. Ethylene also increases the respiration rate of produce, leading to weight loss and associated profits. This is also true for climacteric fruits. So, when non-climacteric produce is stored, ethylene levels are monitored to keep them between 0.03-0.38 ppm. Even climacteric fruits storage time can be extended by 10-30% by storing them below 0.005 ppm until they need to be ripened. Extra ethylene gas is removed by ventilation or scrubbing to keep the produce fresh and increase shelf life.
Ethylene is also monitored when tubers, like potatoes, are stored. Ethylene production due to the use of machines, or the presence of other ethylene producing climacteric fruits, can result in the sprouting of tubers, which reduces their weight or makes them unsuitable for consumption. Detect Fungal Diseases We lose 40-50% of fresh produce due to rotting in the post-harvest stage and about 22.5% of the rotting is caused by fungi. Vegetables and fruits with thin skin are more prone to damage during transport, handling, and storage. The damaged produce provides ideal conditions for the growth of fungal pathogens. Mycotoxins produced by some fungi can result in human health risk.
Ethylene production, which occurs during infestation stress, can be estimated by non-destructive measurement taken precisely, easily, and rapidly by portable devices like the F-900 Portable Ethylene Analyzer. These measurements can be used anywhere in the supply chain, to cull and sort infested commodities. This will limit the spread of produce disease and profit loss.
Since ethylene is regarded as a stress hormone, non-destructive measurement of the gas can be used to check for all pest and disease attacks in the post-harvest stage, as in the following examples:
● It is possible to detect diseases even before physical external symptoms occur in tomatoes and grapes.
● Ethylene produced by an infestation of the fungus Aspergillus flavus in grains like maize can be used to detect the pathogen.
Non-Destructive Ethylene Measurement as a Research Tool Besides large-scale industrial use of ethylene measurements, many research studies use non-destructive measurements of this hormone. Some examples are discussed below to give an idea of how researchers use ethylene detection to make discoveries that have commercial applications.
1. Find sources for medicinal phytochemicals: Ethylene is produced as a result of stress due to wounding and UVB radiation in Opuntia ficus-indica or red prickly pears. The gas acts as a signaling molecule, leading to the production of phenolic compounds of medicinal interest. This research confirmed Opuntia as a new source of phytochemicals, which can be used for producing pharmaceutical and supplementary dietary products.
2. Extend vase-life of flowers: Senescence due to ethylene was measured by a gas analyzer to show that gibberellic acid as a pre-harvest spray can produce larger flowers and keep Peruvian Lily (Alstroemeria hybrida cv. Pink Panther) fresh long after they are cut.
3. Develop stress-resistant cultivars: Ethylene production in response to stress is used to select cultivars that can tolerate or resist different forms of stress like drought, temperature, and salinity. The physiological processes that trigger ethylene production, and even the genes involved, are identified to develop cultivars that will be able to thrive and produce under stress. These kinds of studies are increasing due to global-warming-induced changes in the environment that are expected to impact agricultural and horticultural production. For example, a first-of-its-kind study showed that ethylene influences anthesis in coffee during drought. Suitable Devices Regardless of where ethylene is measured in the agricultural and horticultural supply chains, a precision device is needed that is easy enough for any layperson to use. Luckily, there are a few already on the market.

The F-900 Portable Ethylene Analyzer is an industry standard that is trusted not only by the commercial supply chain but also by research scientists. The F-900 measures between 0-10 ppm of ethylene, with a resolution of 0.001 ppm. It can also measure carbon dioxide and oxygen levels, simultaneously. With this device, stakeholders can get rapid non-destructive measurements in seconds—in shipping containers, controlled atmosphere facilities, and cold storage—and reduce loss of food and flowers to improve their profits.
Sources:
Brecht & Reid. (2010). Ethylene inhibition and control 2010. Retrieved from http://ucce.ucdavis.edu/files/datastore/234-1554....
Chang, C. (2016). Q&A: How do plants respond to ethylene and what is its importance? BMC Biol 14, 7 https://doi.org/10.1186/s12915-016-0230-0
Claver, H. (2021, March 4). "Indoor farming tech market worth $ 24.8 billion by 2026". Future farming. https://www.futurefarming.com/Smart-farmers/Artic...
Cristescu, S.M., Mandon, J., Arslanov, D., et al. (2013). Current methods for detecting ethylene in plants. Ann Bot, 111: 347-360. DOI: 10.1093/aob/mcs259.
Guo, H., Liu, A., Wang, Y., Wang, T., Zhang, W., Zhu, P., & Xu, L. (2020). Measuring light-induced fungal ethylene production enables non-destructive diagnosis of disease occurrence in harvested fruits. Food Chemistry 310. DOI: https://doi.org/10.1016/j.foodchem.2019.125827
Kaviya, S. S., Lourdusamy, K., Ganga, M., & Vincent, S. (2021). Influence of pre-harvest sprays on flower quality and vase life of Alstroemeria. Journal of Pharmacognosy and Phytochemistry, 10(1): 429-433. DOI: 10.22271/phyto.2021.v10.i1Sg.13597
Khan, N. A., Khan, M. I., Ferrante, A., & Poor, P. (2017). Editorial: Ethylene: A Key Regulatory Molecule in Plants. Frontiers in Plant Science, 8. https://doi.org/10.3389/fpls.2017.01782
Leatherwood, W.R., & Mattson, N.S. (2010, February 23). Ethylene In The Greenhouse. Greenhouse Grower. https://www.greenhousegrower.com/production/plant...
Lima, A., Santos, I., Lopez, M., et al. (2021). Drought and re-watering modify ethylene production and sensitivity, and are associated with coffee anthesis. Authorea, 181. DOI: 10.22541/au.158981705.57565156
Moirangthem, K., & Tucker, G. (2018) How Do Fruits Ripen? Front. Young Minds. 6:16. doi: 10.3389/frym.2018.00016
Ortega-Hernández, E., Vimal Nair, V., Jorge Welti-Chanes, J., Luis Cisneros-Zevallos, L., & Jacobo-Velázquez, D.A. (2019). Wounding and UVB Light Synergistically Induce the Biosynthesis of Phenolic Compounds and Ascorbic Acid in Red Prickly Pears (Opuntia ficus-indica cv. Rojo Vigor). Int. J. Mol. Sci., 20(21), 5327; https://doi.org/10.3390/ijms20215327
Symons, G.M., Chua, Y.-J., Ross, J.J., Quittenden, L.J., Davies, N.W., & Reid, J.B. (2012). Hormonal changes during non-climacteric ripening in strawberry, Journal of Experimental Botany, 63(13), 4741–4750. https://doi.org/10.1093/jxb/ers147
Wang, S., Park, Y.-S., Yang, Y., Borrego, E. J., Isakeit, T., Gao, X., & Kolomiets, M. V. (2017). Seed-Derived Ethylene Facilitates Colonization but Not Aflatoxin Production by Aspergillus flavus in Maize. Frontiers in Plant Science, 8. https://doi.org/10.3389/fpls.2017.00415

